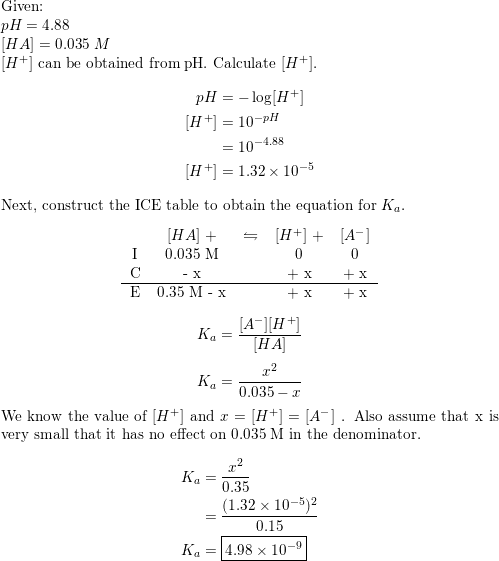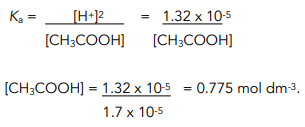A 0.21 M solution of chloroacetic acid, ClCH2CO2H, has a pH of 1.79. Calculate Ka for the acid. | Homework.Study.com
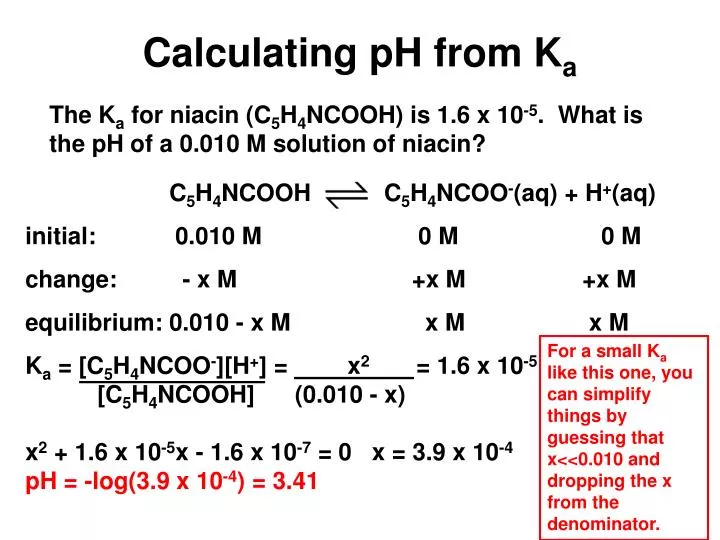
![SOLVED:Calculating [H^+] and p Ka from the pH of a Solution of Weak Acid The pH of a 0.02 M solution of an acid was measured at 4.6. a. What is the [ SOLVED:Calculating [H^+] and p Ka from the pH of a Solution of Weak Acid The pH of a 0.02 M solution of an acid was measured at 4.6. a. What is the [](https://cdn.numerade.com/previews/12327d24-029f-4354-ad85-c698faee7c3c_large.jpg)
SOLVED:Calculating [H^+] and p Ka from the pH of a Solution of Weak Acid The pH of a 0.02 M solution of an acid was measured at 4.6. a. What is the [
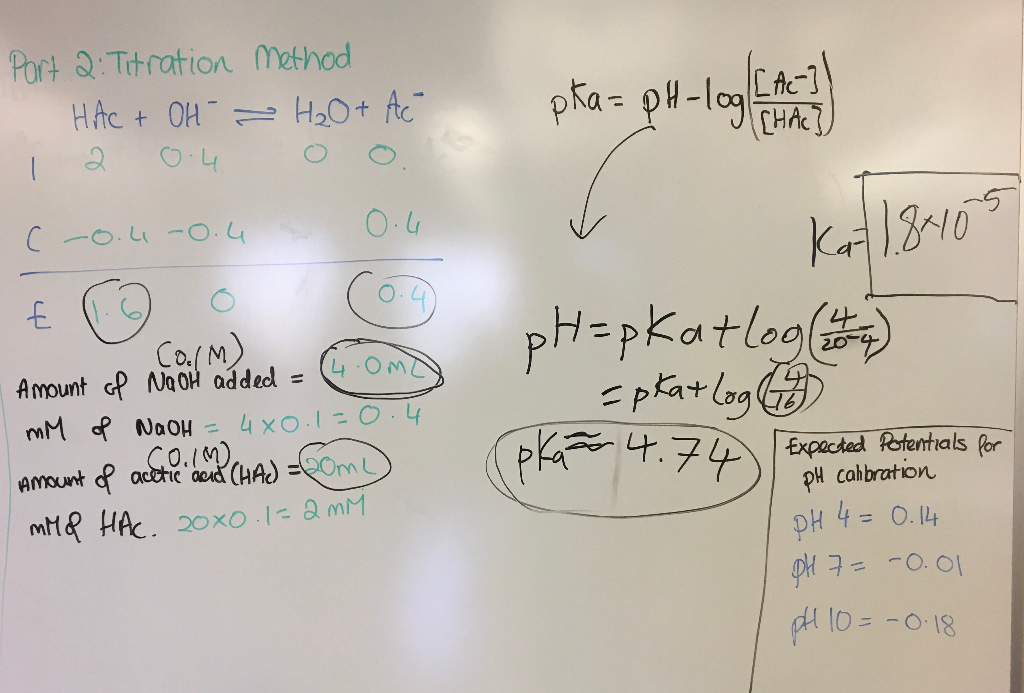
The Ka value for acetic acid, CH3COOH(aq), is 1.8x10^-5. Calculate the ph of a 2.80 M acetic acid solution - Home Work Help - Learn CBSE Forum


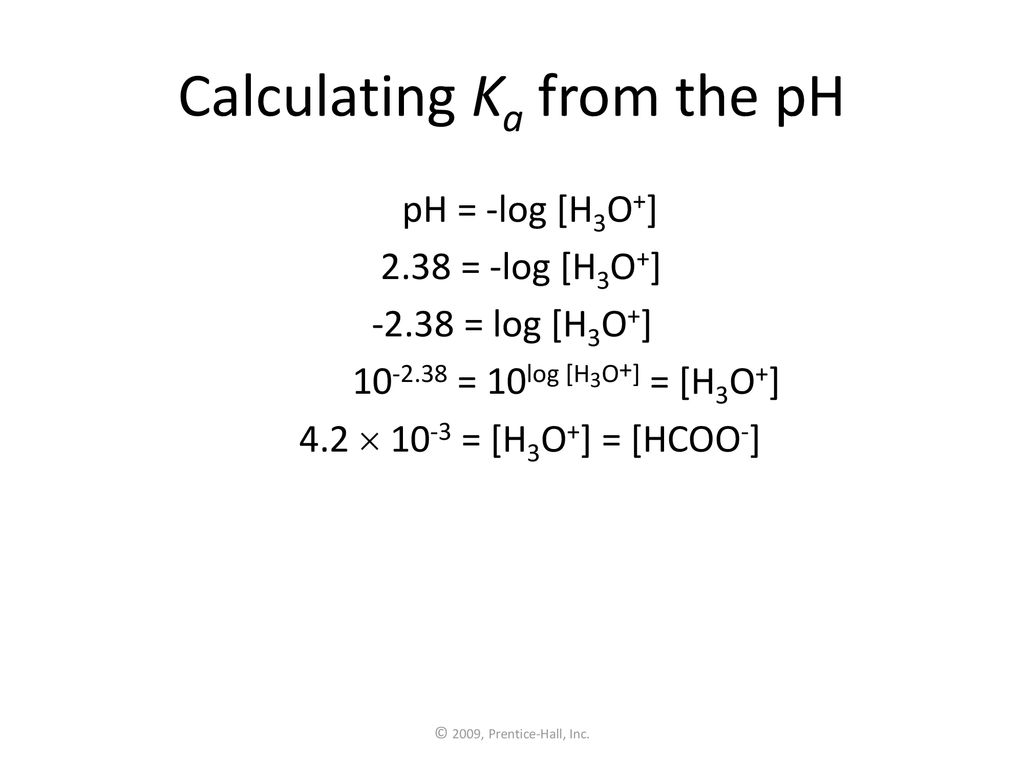
![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)