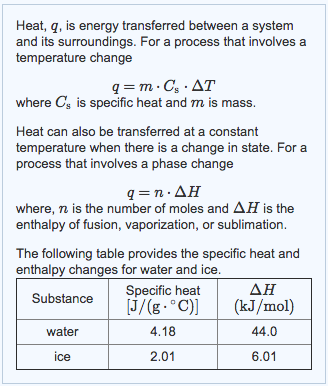
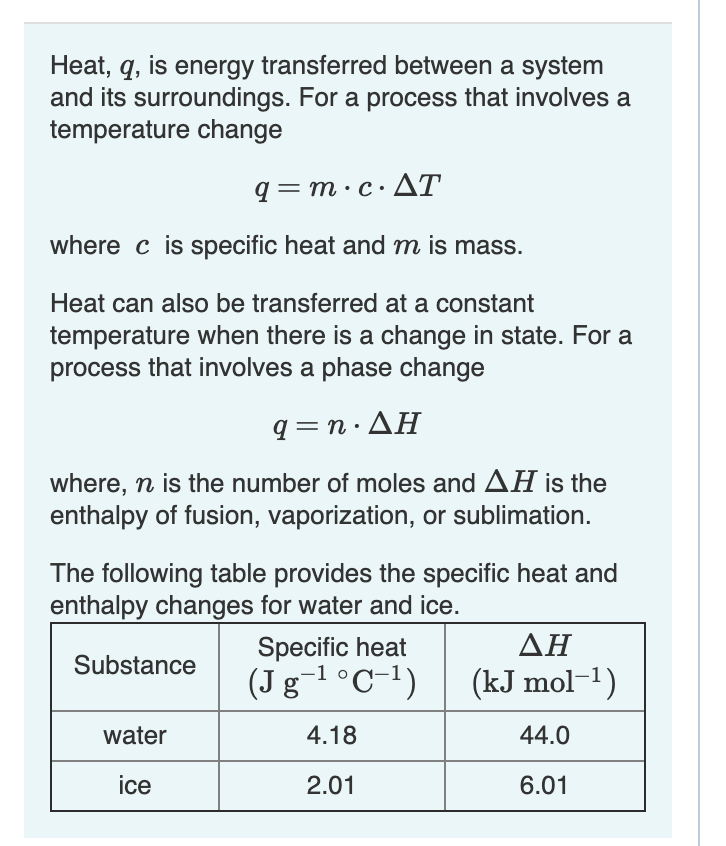
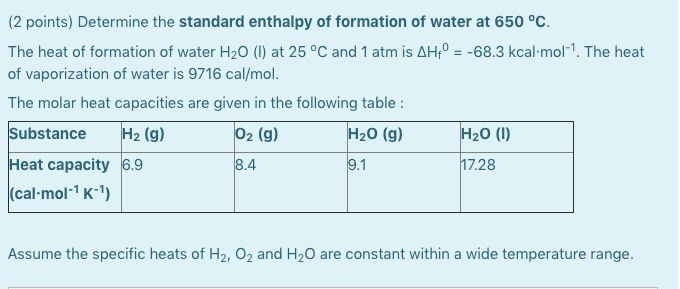
Question Video: Determining a Standard Enthalpy Change Given the Standard Enthlapy of Fusion | Nagwa

Enthalpy Of Water And Steam - Enthalpy of Wet Steam - Enthalpy of Dry And Saturated Steam - - YouTube

The enthalpy change for transition of liquid water to steam is 40.8 kJ `mol^(-1)` at 373K. - YouTube

If water vapor is assumed to be a perfect gas, molar enthalpy change for vaporization of 1 mol of... - YouTube

Calculate the enthalpy change on freezing of `1 mol` of water at `10^(@)C` to ice at `-10^(@)C`. - YouTube

Calculate change in enthalpy when 2 moles of liquid water at 1 bar and `100^(@)` C is coverted into - YouTube

Calculate the enthalpy of formation of water, given that the bond energies of `H-H, O=O` and `O-... - YouTube

Question Video: Calculating the Enthalpy of Dilution of Sodium Hydroxide Using Enthalpy of Solution Values | Nagwa