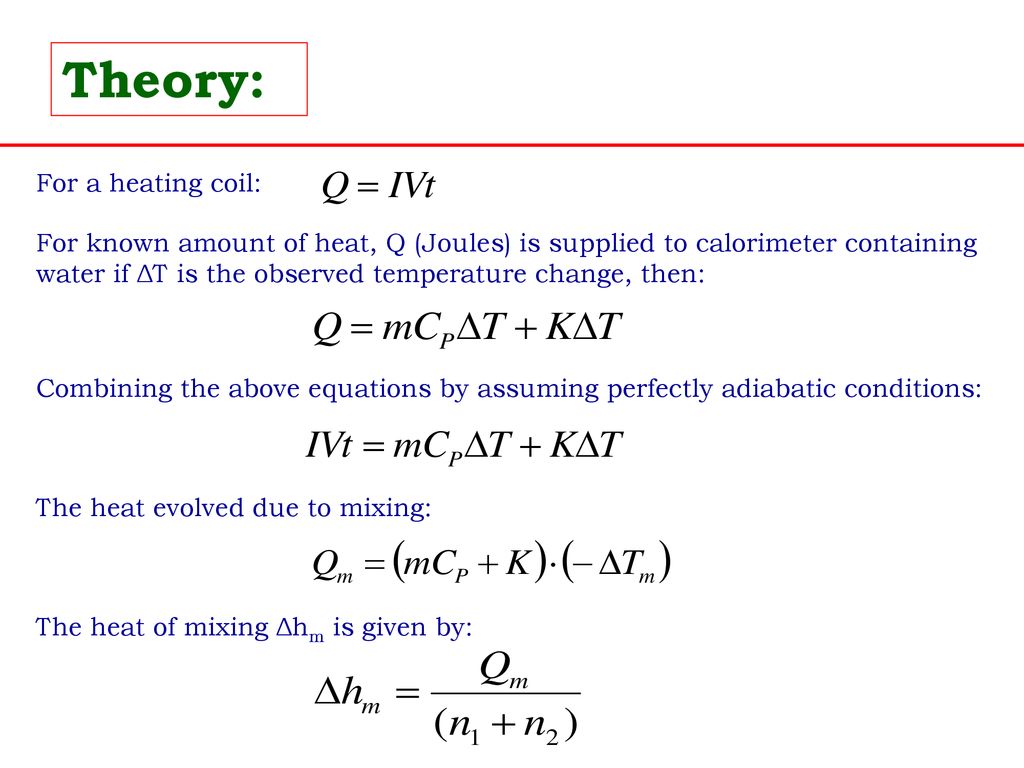
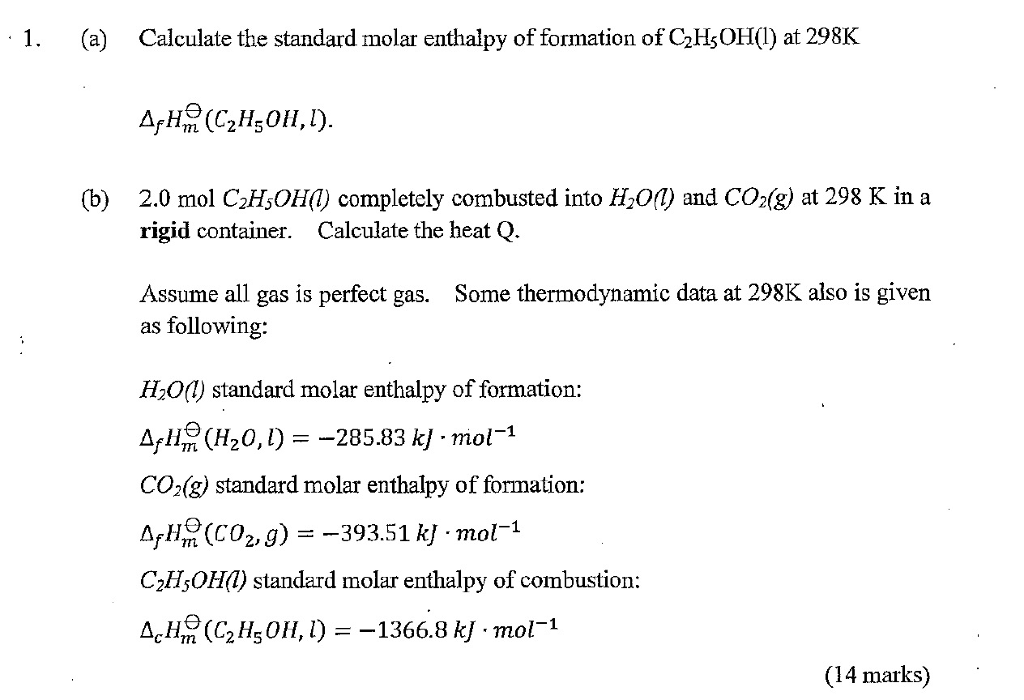
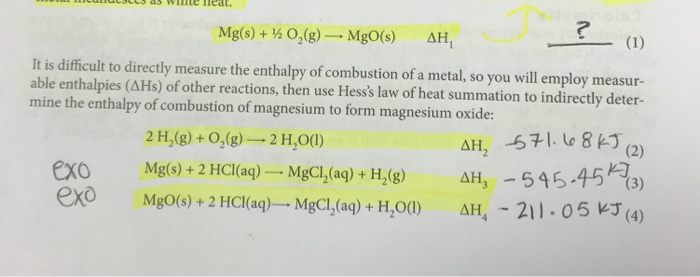
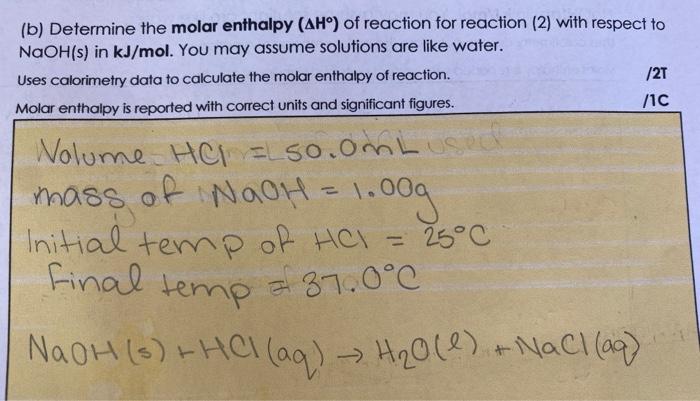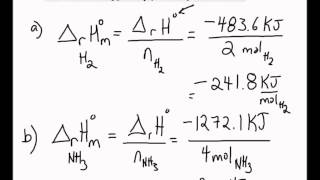Molar Enthalpies. use proper scientific terminology to describe molar enthalpies calculate molar enthalpies Calculate molar enthalpies using the. - ppt download
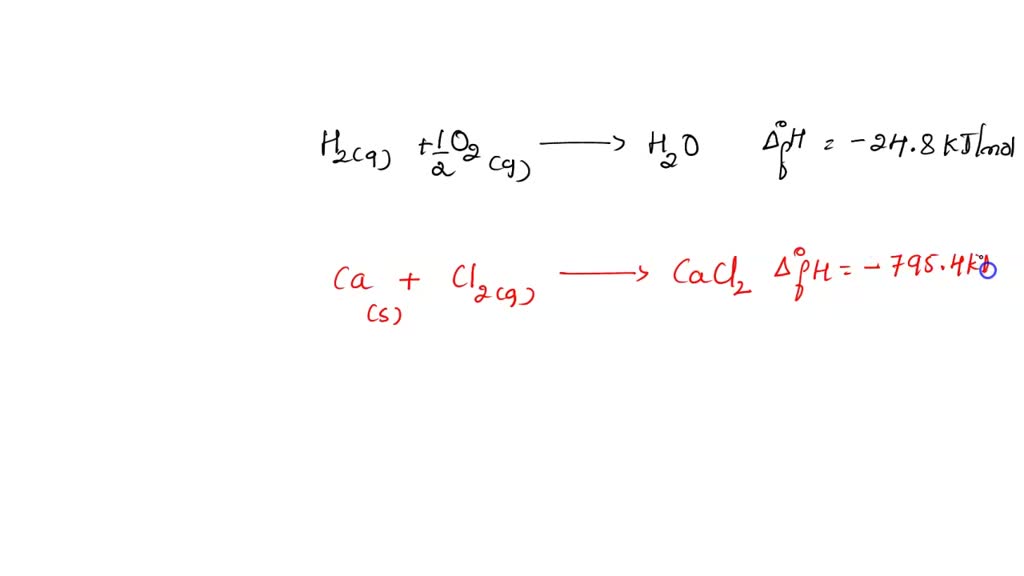
SOLVED: Write a balanced thermochemical equation to represent the standard molar enthalpy of formation of each of the following substances. Include the heat term within the equation. (a) H2O(g) [The reactants are

Calculating the Heat of Reaction from Molar Reaction Enthalpy and the Mass of a Reactant | Chemistry | Study.com
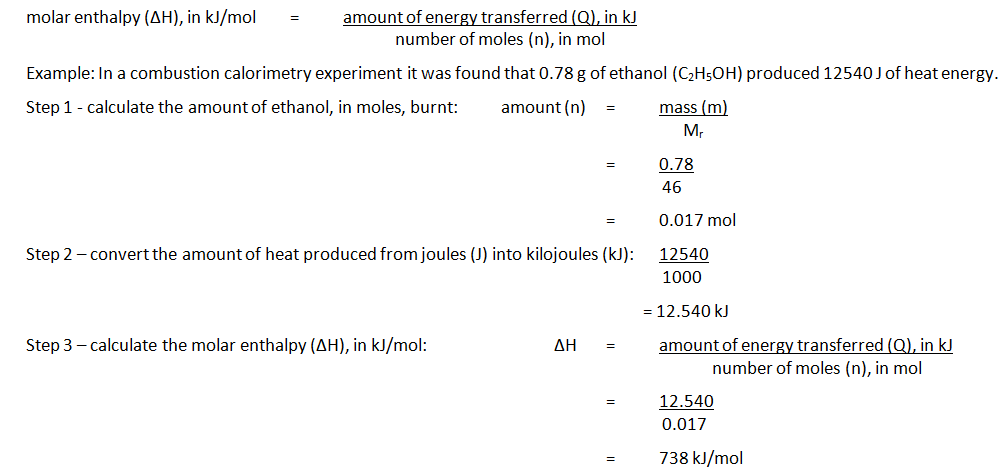
3:04 calculate the molar enthalpy change (ΔH) from the heat energy change, Q - TutorMyself Chemistry

The molar enthalpy change for H2O(1) H2O(g) at 373 K and 1 atm is 41 kJ/mol. Assuming ideal behavior, the internal energy change for vaporization of 1 mol of water at 373